NeuroStar® TMS Therapy
FDA-cleared, non-medication depression treatment
Existing Patients For Providers →
(855) 940-4867
Still struggling with depression after trying antidepressants and talk therapy? Greenbrook Mental Wellness Centers Glen Burnie is a trusted provider of innovative treatment options for individuals with depression, including NeuroStar® Transcranial Magnetic Stimulation (TMS) therapy and SPRAVATO® (esketamine) nasal spray. Most major insurance plans cover our treatments. Schedule a no-cost consultation with a member of our Care Team to find out if these therapies are right for you.
info@greenbrooktms.com
(855) 940-4867
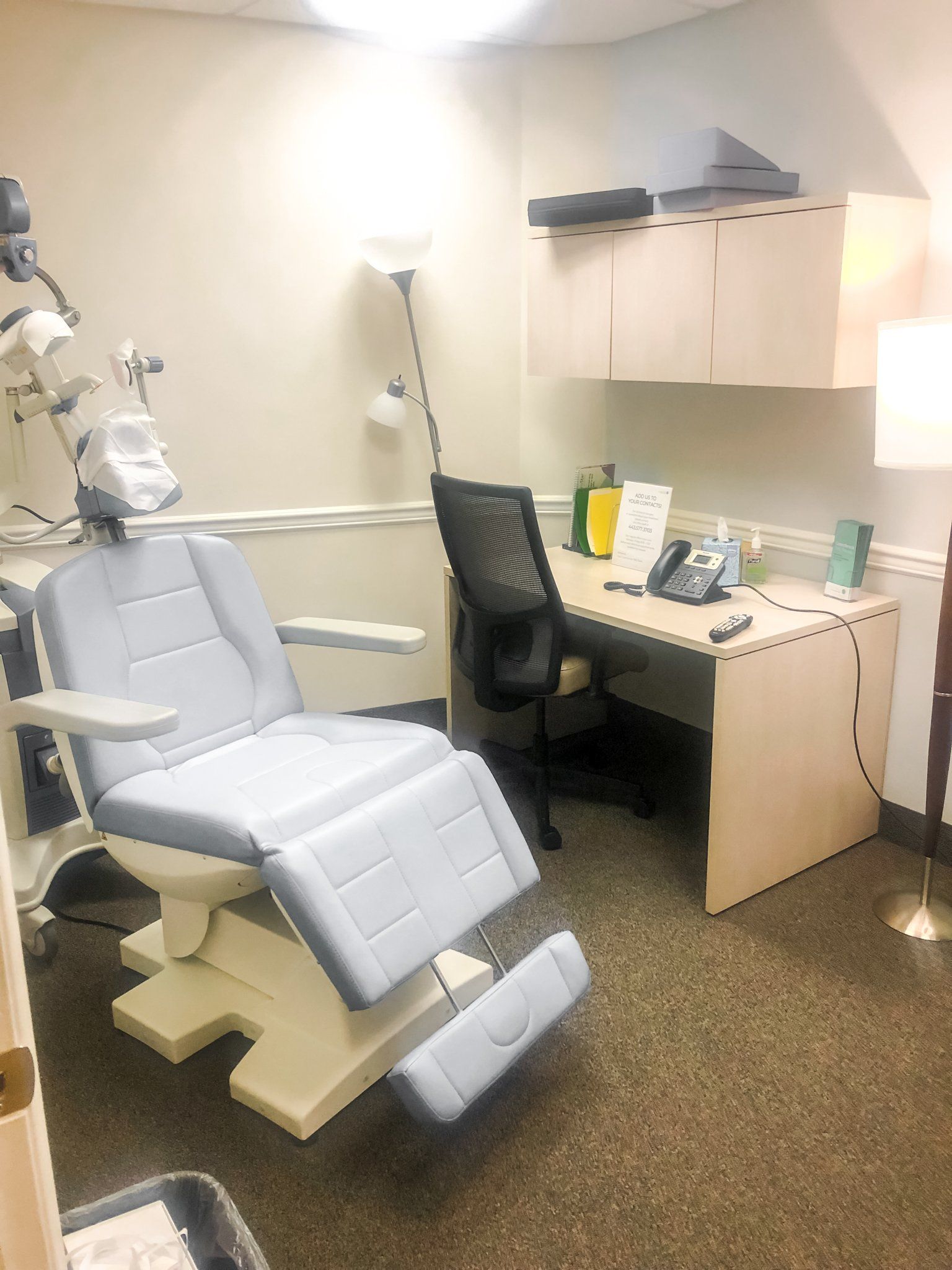
1600 Crain Highway S.
Suite 602
Glen Burnie, MD 21061
Holiday weekend, and extended hours by appointment
Located in Crain Towers on Rt. 3, just off Rt. 100, and 1 mile from Baltimore Washington Medical Center. Head south on Route 3, and the towers will be on your first sharp right turn following Crabtown and across the street from Hardees. Parking is open. Enter through the front and take the elevators to the 6th floor. Suite 602 is directly on your lefthand side.
At Greenbrook our dedicated Care Team will support you every step of the way—from insurance coordination to scheduling and beyond. Schedule a no-cost consultation today, and we'll help you find a depression treatment that's right for you. We're here to answer any questions you have.
During your consultation, you will meet with a Consult Coordinator to learn about the treatment process, and our Care Team will start the insurance paperwork if needed. Next, you will meet with a Greenbrook-affiliated licensed mental health provider for an evaluation, which includes detailed questions about your history and symptoms. If the Greenbrook provider has determined that you're a candidate for SPRAVATO® or NeuroStar TMS therapy and you're ready to move forward, they will create your individualized treatment plan. Then, you're ready to start treatment at Greenbrook!
More about NeuroStar TMS therapy
What is NeuroStar TMS therapy?
NeuroStar TMS (Transcranial Magnetic Stimulation) goes right to the source of depression – your brain. It is a non-invasive, non-drug treatment that uses focused magnetic pulses to “wake up” those areas and help your brain work the way it should. It’s like physical therapy for your brain.1,2
What is the average NeuroStar TMS treatment plan?
Greenbrook NeuroStar TMS treatment plans typically include 30 to 36 sessions over a six- to nine-week period, depending on the patient’s diagnosis, insurance requirements, and individualized care plan. Each session is as little as 19 minutes, and you can resume normal activities immediately after treatment.
More about SPRAVATO® (esketamine) nasal spray treatment
What is SPRAVATO®?
SPRAVATO® is an FDA-approved nasal spray. It is a prescription medicine used:
What is a typical SPRAVATO® treatment plan?
Treatment is provided under the direct supervision and monitoring of trained medical professionals for at least two hours. After your two-hour monitoring, you may feel groggy and woozy. You will need to avoid hazardous activities for the remainder of the day, including operating a motor vehicle.
A typical treatment plan consists of:
Treatment plans may vary depending on clinical conditions.
1. Post A, et al. (2001). J Psychiatric Research, 35:193-215.
2. Liston C, Chen AC, Zebley BD, et al. (2014). Biol Psychiatry, 75(7);517-526.







<p class="rteBlock">Maryland</p>
<p class="rteBlock">Glen Burnie</p>
Please provide the requested information on the form and Greenbrook Mental Wellness Centers will contact you shortly. All information will be kept strictly confidential. We do not sell or share your information.
*If you are a referring Physician interested in contacting Greenbrook Mental Wellness Centers about a current patient, please use this form.
Greenbrook supports an accessible internet. If you have any questions about our accessibility features, please contact us at
(855) 940-4867 or info@greenbrooktms.com.
All Rights Reserved | Greenbrook TMS NeuroHealth Centers.