NeuroStar® TMS Therapy
FDA-cleared, non-medication depression treatment
Existing Patients For Providers →
(855) 940-4867
Greenbrook offers SPRAVATO® (esketamine) nasal spray, the first and only monotherapy for adults with treatment-resistant depression.
SPRAVATO® is an FDA-approved esketamine nasal spray used to treat adults with treatment-resistant depression (TRD) and depressive symptoms in those with major depressive disorder (MDD) who have suicidal thoughts or actions. Approved in 2019, it is taken with or without an oral antidepressant for TRD and alongside an oral antidepressant for MDD with suicidal thoughts or actions.
You will first talk with one of our intake specialists, who will collect some basic information and arrange a meeting with one of our Consult Coordinators. The Consult Coordinator can meet with you in person, over video conference, or on the phone and will review your history and explain aspects of esketamine nasal spray treatment.
Your Care Team will also explain treatment payment options and review standard insurance plan criteria with you. We will coordinate with your health insurance provider to determine benefits.
IS SPRAVATO® COVERED BY INSURANCE?
Yes! Many major insurance companies cover SPRAVATO® if you haven't found adequate relief after trying just TWO oral antidepressants.
A benefit of having treatment at a Greenbrook center is that our Care Team does all the work for you regarding insurance. We assist all patients with the coverage and reimbursement process.
SHOULD I RECEIVE IV KETAMINE OR SPRAVATO® (NASAL ESKETAMINE)?
IV ketamine
SPRAVATO® (esketamine) nasal spray
Next, you will meet with a Greenbrook affiliated provider for an evaluation, which includes questions about your history and symptoms.
If the Greenbrook provider has determined that you are a candidate for esketamine nasal spray and you are ready to move forward, they will create your individualized treatment plan.
At most Greenbrook centers, we try to book your
SPRAVATO® treatments to accommodate your schedule, whether morning or afternoon appointments work best for you.
After you arrive at the center, you will be placed in a reclining chair where you can sit back and relax. You will be handed a nasal spray device to self-administer. Your care team will supervise you for at least two hours. Afterward, you will need someone to drive you home from treatment. By the next morning, most people are ready to resume their day with no restrictions.
TYPICAL SPRAVATO®
TREATMENT SESSION

During SPRAVATO® treatment, you may feel dizzy or euphoric. Our Care Team will be there to assist you throughout your treatment. Your blood pressure will be checked upon arrival, 40 minutes in, and before you leave.
After your two-hour treatment, you may be groggy and woozy. You will need to avoid hazardous activity for the remainder of the day, including operating a motor vehicle, so you will need someone to drive you home from treatment.
Treatment plans may vary depending on clinical conditions.
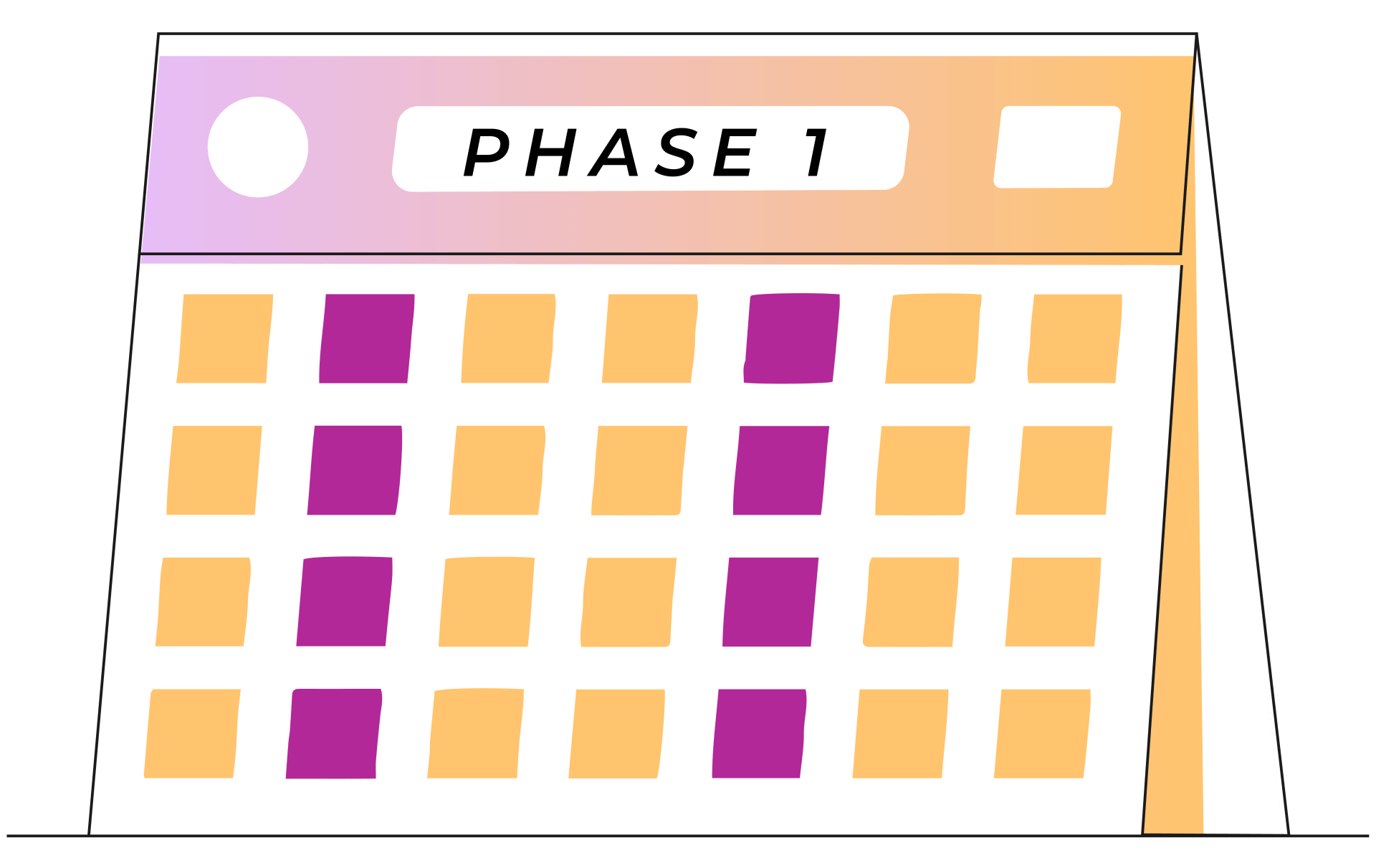
Week 1-4
2 treatments each week
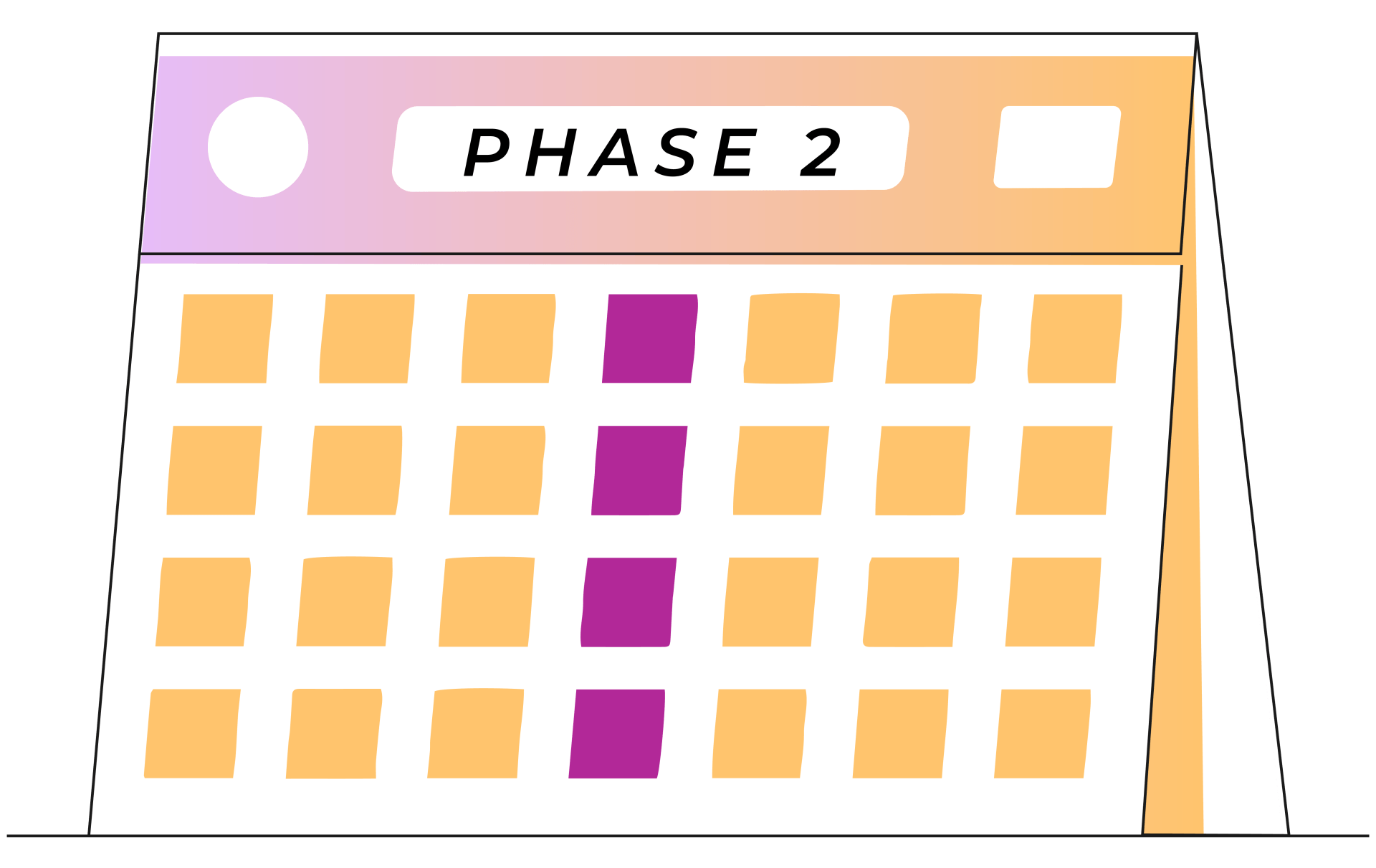
Week 5-8
1 treatment each week
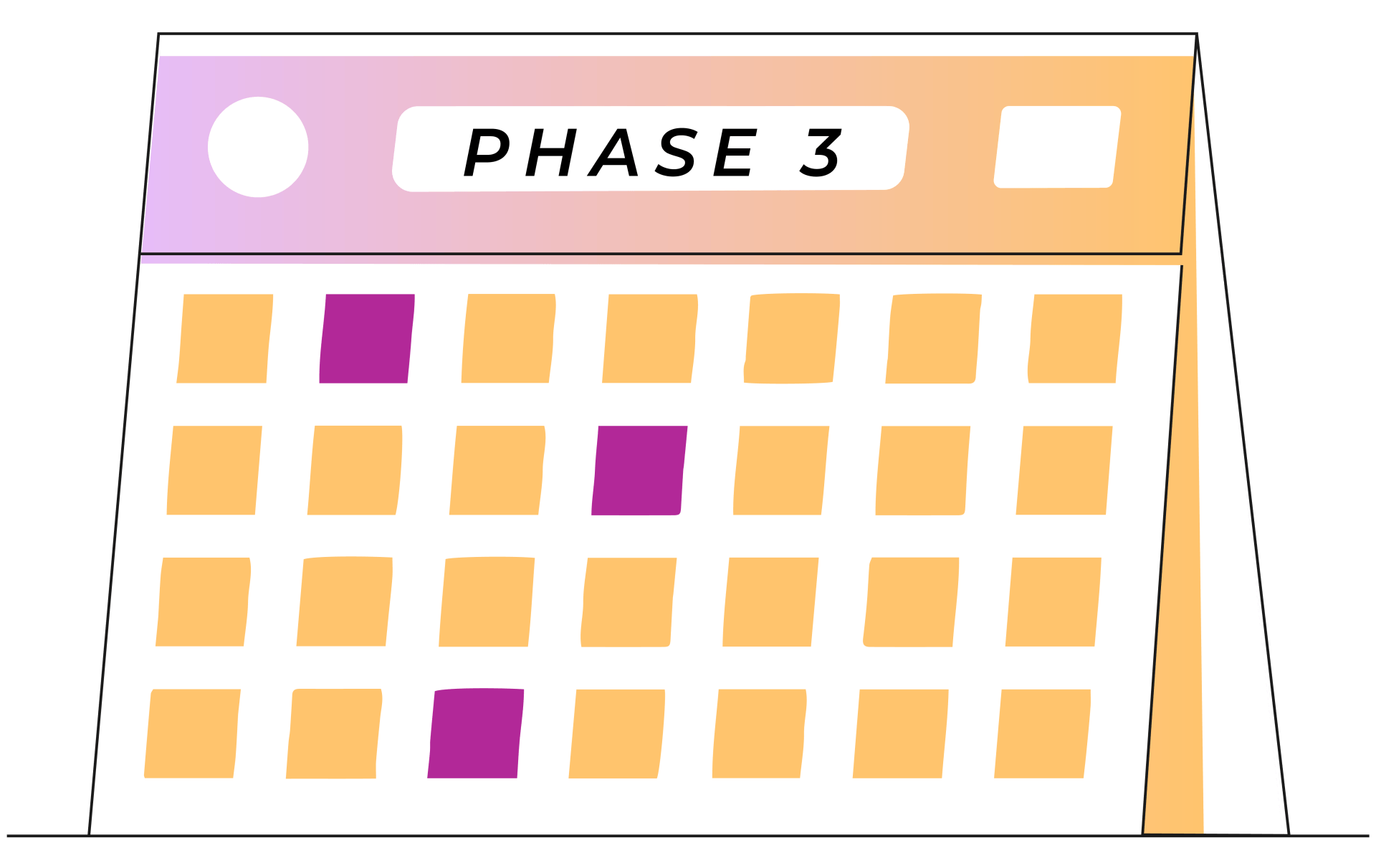
Week 9+
1 treatment every 1-2 weeks
Your Consult Coordinator will help you find out if SPRAVATO® is right for you!

Please provide the requested information on the form and Greenbrook will contact you shortly. All information will be kept strictly confidential. We do not sell or share your information.
SPRAVATO® is an FDA-approved esketamine nasal spray, a refined form of ketamine, used to treat adults with treatment-resistant depression and depressive symptoms in adults with major depressive disorder (MDD) with suicidal thoughts or actions. It is also covered by most major insurances.
Unlike SPRAVATO®, ketamine is not FDA-approved or covered by many insurances.
SPRAVATO® Is an FDA-approved esketamine nasal spray used to treat adults with major depressive disorder who have not received adequate relief after two or more oral antidepressant trials.
A typical round of esketamine nasal spray treatment is broken up into three phases:
Treatment plans may vary depending on clinical conditions.
Before each appointment, avoid eating for two hours and drinking large amounts of fluid for 30 minutes.
Each session takes place at a private, spa-like Greenbrook center. Once you arrive, you'll sit in a comfortable recliner, and a technician will check your blood pressure. You will then complete a short form, after which you will be handed a nasal spray device to self-administer. We will go over how to administer the spray to yourself.
Throughout your treatment, our Care Team will be onsite to assist you. Your blood pressure is checked at the start of the session, 40 minutes into treatment, and before you leave the Greenbrook center. You must stay in the center for at least two hours. Ensure you have someone to drive you home, as you will not be able to operate a motor vehicle for the rest of the day.
Your Greenbrook provider will determine the dose of SPRAVATO® based on your treatment plan. There will be 2-3 sprays self-administered in each nostril, depending on the dose prescribed by your provider.
That's okay! Our Care Team will work with you to determine if SPRAVATO® depression treatment is the right choice. If not, Greenbrook also offers NeuroStar TMS therapy—an FDA-cleared, non-drug, and non-sedating treatment for depression. Our psychiatric professionals are here to help you find the best approach for your needs. You can learn more about NeuroStar TMS by asking your Care Team or taking our TMS quiz.
Yes. SPRAVATO® can be administered as a stand-alone therapy or combined with other oral antidepressant medications. Our psychiatric professionals will work with you to determine the best approach for your needs.
SPRAVATO® is available through the SPRAVATO® Risk Evaluation and Mitigation Strategy (REMS) Program at convenient outpatient Greenbrook Mental Wellness Centers.
The medical staff at Greenbrook’s REMS-certified outpatient treatment centers are trained to prescribe and observe the patient's self-administration of SPRAVATO®.
No. Esketamine nasal spray is FDA-approved for adults with major depressive disorder who have not experienced adequate improvement with antidepressant medications.
Greenbrook Mental Wellness recommends calling for more information on whether treatment is right for you. Below are some of the common contraindications, but each patient's journey is unique.
Yes! Many major insurance companies cover SPRAVATO® depression treatment if you haven’t found adequate relief after trying two oral antidepressants.
Greenbrook supports an accessible internet. If you have any questions about our accessibility features, please contact us at
(855) 940-4867 or info@greenbrooktms.com.
All Rights Reserved | Greenbrook TMS NeuroHealth Centers.